Nacl Lewis Structure / Ninth Grade Lesson Ionic Bonding With Lewis Dot Diagrams - An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.
Nacl Lewis Structure / Ninth Grade Lesson Ionic Bonding With Lewis Dot Diagrams - An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.. Where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. Sodium chloride has the formula nacl. Sodium chloride nacl lets take a moment to go over the rules for lewis dot structures. When depleted in the body, sodium must be replaced in order to maintain intracellular osmolarity, nerve conduction, muscle contraction and normal renal function. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.
Lewis dots are named after gilbert lewis, a chemist who studied how elements bond, or attach together. In the chloride ion, the outer shell of valence electrons is complete with 8 electrons. Draw lewis structure of hcooh lewis dot structure of hco2h formic or methanoic acid roco lewis drawing w patt. The lewis structure for the salt nacl shows two ions which have their now outer shells of electrons filled with a complete octet. Administration of urea elevates blood plasma osmolality, resulting in enhanced flow of water from tissues, including the brain, cerebrospinal fluid and eye.
Bond, molecular geometry, lewis structure, linear, trigonal planar, bent, tetrahedral, trigonal pyramidal:
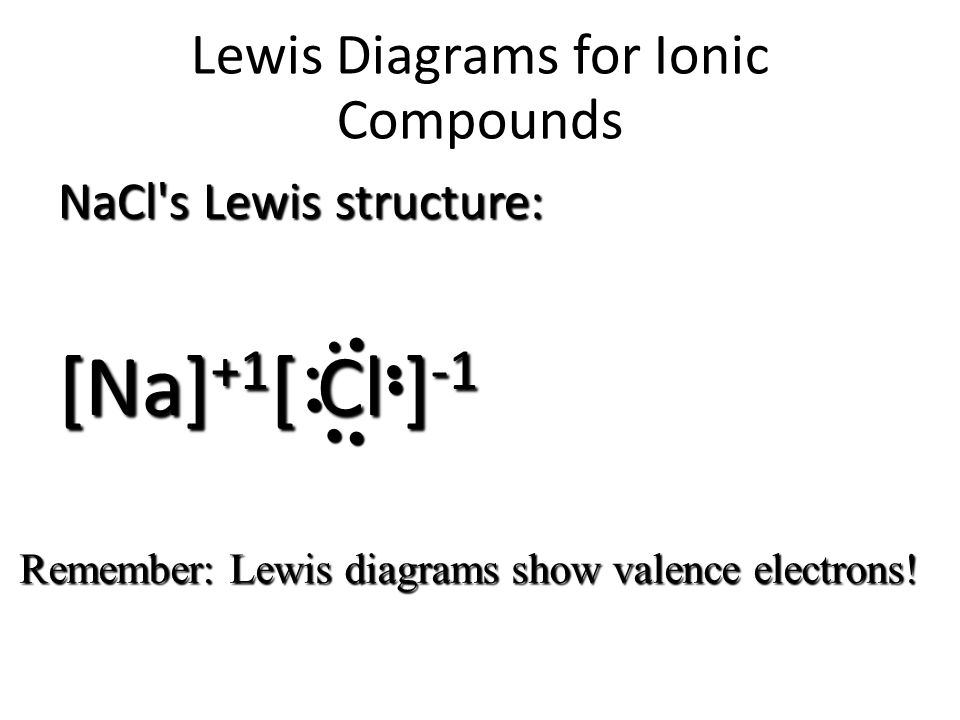
The lewis dot structure of nacl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Draw lewis structure of hcooh lewis dot structure of hco2h formic or methanoic acid roco lewis drawing w patt. The oxidation of sodium metal; The lewis structure for the salt nacl shows two ions which have their now outer shells of electrons filled with a complete octet. To satisfy the octet of carbon, one of the pairs of electrons on oxygen must be moved to create a double bond with carbon. Bond, molecular geometry, lewis structure, linear, trigonal planar, bent, tetrahedral, trigonal pyramidal: The lewis structure is drawn in such a way that the octet of each atom is complete. The lewis structure for the salt nacl shows two ions which have their now outer shells of electrons filled with a complete octet. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. The reaction can be thought of involving the following simultaneous processes: For kcl we have an ionic compound and we need to take that into account when we draw the lewis structure. Administration of urea elevates blood plasma osmolality, resulting in enhanced flow of water from tissues, including the brain, cerebrospinal fluid and eye. The formation of sodium chloride involves sodium metal, na and chlorine gas, cl 2.
683 hazardous substances data bank (hsdb) sodium chlorite is a strong oxidizing agent and under proper reducing conditions is readily reduced to chloride , and to a lesser extent, chlorate. As you can see chlorine.since sodium is a metal, it has relatively low values for ionization energy and electronegativity. Lewis electron dot symbol to describe ionic bonding in sodium chloride. The lewis dot structure for francium. We'll first draw the metal and put it in brackets.

Sodium chloride has the formula nacl.
The lewis structure for the salt nacl shows two ions which have their now outer shells of electrons filled with a complete octet. To satisfy the octet of carbon, one of the pairs of electrons on oxygen must be moved to create a double bond with carbon. 683 hazardous substances data bank (hsdb) sodium chlorite is a strong oxidizing agent and under proper reducing conditions is readily reduced to chloride , and to a lesser extent, chlorate. The lewis structure for the salt nacl, shows two ions which have their (now) outer shells of electrons filled with a complete octet. We'll first draw the metal and put it in brackets. Structure, properties, spectra, suppliers and links for: Sodium chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. As you can see chlorine.since sodium is a metal, it has relatively low values for ionization energy and electronegativity. Lewis dots are named after gilbert lewis, a chemist who studied how elements bond, or attach together. In vivo, urea is formed in the liver via the urea cycle from ammonia and is the final end product of protein metabolism. The lewis structure is drawn in such a way that the octet of each atom is complete. When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Is the diagram a correct lewis structure of sodium chloride?
Lewis electron dot symbol to describe ionic bonding in sodium chloride. Urea is a nitrogenous compound containing a carbonyl group attached to two amine groups with osmotic diuretic activity. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells. And hence several thousands times slower than electrons). The charges add up to the overall charge of the ion.
As you can see chlorine.since sodium is a metal, it has relatively low values for ionization energy and electronegativity.
For kcl we have an ionic compound and we need to take that into account when we draw the lewis structure. In the chloride ion, the outer shell of valence electrons is complete with 8 electrons. When depleted in the body, sodium must be replaced in order to maintain intracellular osmolarity, nerve conduction, muscle contraction and normal renal function. Is the diagram a correct lewis structure of sodium chloride? A) the diagram is a correct lewis structure of methane na+t:01: Thus, we write the lewis structure for nacl as: As you can see chlorine.since sodium is a metal, it has relatively low values for ionization energy and electronegativity. This means that sodium loses an electron to achieve the stable noble gas configuration of 8 valence electrons. Normal valence, we call it the hypervalence. B) the diagram is an incorrect lewis structure of methane. Na is in the first column of the periodic table so its na+. The lewis dot structure of nacl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.

Komentar
Posting Komentar